Mapping Function Through Architecture: The Essence of Spatial Medicine
From Sequences to Spatial Context
The Loss of Context in Tissue Biology: Evolution of Genomic Platforms
Our understanding of tissue biology has advanced through successive technological revolutions, each driven by the pursuit of higher genomic resolution.
Initially, bulk RNA-seq provided averaged gene expression profiles across large tissue masses. While this approach was foundational for uncovering global expression programs and genetic alterations, it inherently obscured the cellular heterogeneity and intercellular communication that define tissue function (1).
The emergence of single-cell RNA sequencing (scRNA-seq) transformed cellular taxonomy by offering single-cell resolution, enabling the identification of rare states, subclonal populations, and cell-type–specific transcriptional programs (1). However, this method requires tissue dissociation into single-cell suspensions, leading to a critical loss of architectural and contextual information in situ (1).
Spatial transcriptomics (ST) has emerged as a response to this limitation, integrating the molecular richness of the transcriptome with the indispensable architectural and microenvironmental context of the tissue (2). This technology validates a central principle: cell function arises from spatial positioning and local interactions. In doing so, it redefines biology, shifting from a linear paradigm to an ecological one (2).
Why the Spatial Dimension Redefines Tissue Biology (Contextual Oncology)
Spatial organization is not a mere morphological detail, it is a primary functional regulator of disease progression. The position of cells within specific niches, such as the invasive front, hypoxic zones, or immune-rich microenvironments, directly dictates their phenotype, transcriptional programs, and therapeutic response (1).
Imagine this:
You have two football teams competing in the World Cup, eleven players against eleven. Now place all twenty-two players in a five-square-meter room and throw in a ball. Technically, the setup is the same: same players, same ball, same goal of scoring. So what could go wrong?
If you know anything about football, you’d think this scenario is absurd. And that’s precisely the point, the space is what makes the game possible. Without space, there are no dynamics, no strategy, no flow, and no way to interpret movement, possession, or creativity. Everything that makes football beautiful, the passes, the structure, the interplay, emerges from how players occupy and use space.
Now, imagine the same principle in biology. Cells, like players, don’t exist in isolation, they act, react, and adapt within a spatial field of interactions. Remove the spatial context, and you lose the essence of the biological “game.” This is exactly what spatial transcriptomics brings back to the field: it allows us to see not just who the players are, but how and where they play together to create the living system.
Spatial transcriptomics, therefore, is the engine of Contextual Oncology. It restores molecular discoveries to their native tissue architecture, revealing that key processes (drug resistance, immune evasion, cellular differentiation) are not random molecular events but spatially regulated biological dynamics (1).
Integration with Digital Pathology and Contextual Medicine
Digital pathology, built upon whole-slide imaging (WSI) of H&E-stained tissues, provides a rich and scalable morphological foundation (1). ST complements this visual layer by overlaying quantitative molecular data that validate and qualify histological features traditionally assessed qualitatively, such as desmoplasia, tertiary lymphoid structures, or invasive front composition (1).
The integration of ST and H&E, increasingly powered by artificial intelligence (AI), accelerates the transition from purely morphological histopathology to data-driven, contextual diagnosis. This synergy is pivotal for clinical adoption, as it offers a natural and intuitive extension of existing histology workflows, workflows that pathologists are inherently trained to interpret (1).
Once we recognize that biology is spatial by nature, we face an unavoidable question: how can we measure the invisible architecture that sustains life? The answer lies in computation. Mathematics, graphs, and algorithms become our instruments to decode the tissue’s spatial language and turn qualitative intuition into quantitative insight
Foundational Models and Conceptual Frameworks
The Computational Imperative of Spatial Transcriptomics
The inherent complexity of spatial transcriptomics (ST) data demands advanced computational frameworks. ST datasets present unique challenges such as data sparsity, particularly pronounced in subcellular-resolution or panel-limited platforms (iST), and in low-resolution sequencing-based technologies (55 μm), where multiple cell types are often mixed within a single capture spot, creating the classic deconvolution problem (1).
Moreover, integrating high-resolution histological images (WSI) with millions of transcriptomic coordinates imposes significant computational demands (3). Addressing this challenge requires architectures capable of combining spatial proximity, gene expression, and morphological information into unified analytical models.
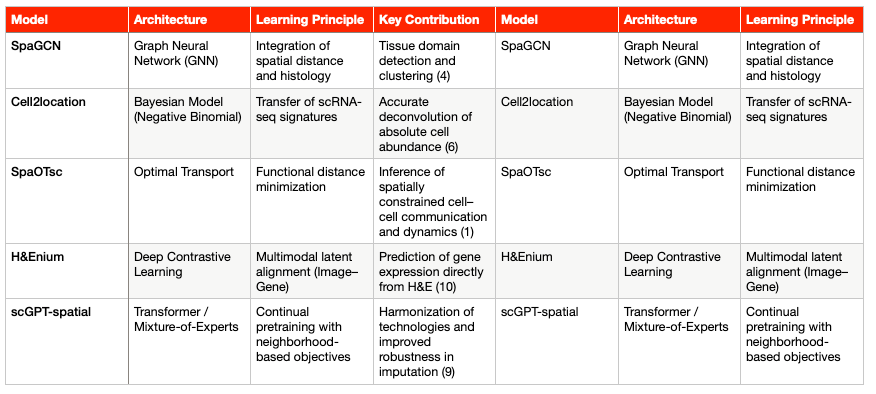
Graph-Based and Contrastive Learning Models
Modeling tissues as graphs, where nodes represent cells or capture spots and edges represent spatial proximity, provides a powerful framework to decipher functional communication and tissue architecture (4).
SpaGCN and GraphST: These models employ Graph Neural Networks (GNNs) to refine gene expression information by integrating it with local spatial and morphological context. The ability of GNNs to weigh information from neighboring nodes is critical for accurately detecting tissue domains and refining segmentation (4). In particular, GraphST can reconstruct cell-type–specific expression patterns (a form of deconvolution) to reveal cellular states within low-resolution spots (4).
Contrastive Learning (CLPLS): Methods that incorporate Graph Contrastive Learning (GCL), such as CLPLS, enhance robustness in key tasks like cellular deconvolution. By applying augmentations at both the node and subgraph level, GCL increases model sensitivity to fine-scale transcript interactions and larger spatial patterns. This feature is essential to overcome the data sparsity typical of ST (5).
Together, these approaches mark a shift from linear statistical modeling to context-aware spatial computation, where proximity and morphology are treated as integral dimensions of biological meaning.
Deconvolution, Mapping, and Optimal Transport Models
Cell2location: A Bayesian regression model (Negative Binomial) designed to infer absolute cell abundance and spatial localization from reference scRNA-seq profiles (6). Its statistical rigor allows it to increase sensitivity and resolution by borrowing strength across spatial locations, guided by prior expectations of cell-type abundance (7).
Tangram: A deep learning framework that uses cost-minimization principles to align scRNA-seq data (which lack spatial context) with tissue coordinate maps. This computationally efficient alignment effectively resolves the core problem of tissue dissociation (5).
SpaOTsc (Spatial Optimal Transport): Based on optimal transport algorithms, this model estimates the functional distance between cells or capture spots. Its key applications include the inference of spatially constrained cell–cell communication, dynamic cell transitions, and intercellular regulatory flows (1). SpaOTsc serves as the computational foundation for ligand–receptor interaction analyses within functional niches (see Section 5).
These models form the analytical backbone of spatial biology, linking molecular expression to spatial logic through mathematical precision.
Emerging Foundation Models for Spatial Transcriptomics (ST-FMs)
To address the lack of standardization and scalability, a new generation of Foundation Models (FMs) is emerging. These models are designed to harmonize heterogeneous data from diverse platforms and omic modalities. Given the scarcity of large ST datasets, even the largest available Visium collection (N = 515) remains small compared to digital pathology repositories(8). Consequently, these FMs leverage knowledge transfer from adjacent domains.
scGPT-spatial: Based on a Transformer architecture, this model employs continual pretraining on large spatial datasets. To accommodate diverse sequencing protocols (sST, iST), it uses a Mixture-of-Experts (MoE) structure in its decoders (9). The goal is to harmonize technologies, improve robustness in gene-expression imputation, and enhance performance in clustering and deconvolution tasks (9).
H&Enium and MISO: These deep learning models are designed for multimodal alignment between pathology images (H&E) and genetic data. Using a Contrastive Alignment framework, they project embeddings derived from foundational pathology and genomic models into a shared latent space (10). This approach demonstrates that tissue morphology encodes predictive information about the transcriptome that can be extracted by AI. As a result, gene-expression profiles can now be predicted at near–single-cell resolution directly from routine H&E slides (3).
Every model is only as strong as the data that sustain it. After exploring how spatial biology is being modeled mathematically, the next step is to understand where these data come from the experimental technologies that make it possible to map gene expression in space. These platforms are not just tools, they define what we can and cannot see in tissue biology.
Core Methodologies and Data Modalities
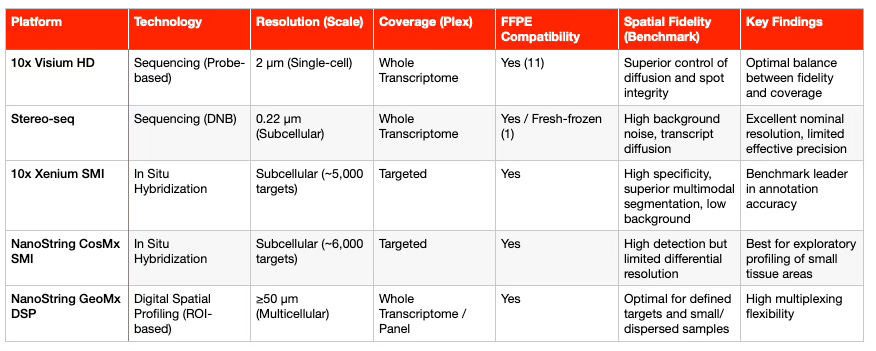
The technological landscape of spatial transcriptomics (ST) can be categorized by the balance it achieves between transcriptome coverage (Whole Transcriptome Analysis, WTA) and spatial resolution (single-molecule).
Capture- and Sequencing-Based Platforms (sST)
These technologies use spatially barcoded arrays to capture transcripts across tissue sections.
10x Visium (Original, 55 μm): Offers multicellular resolution, with each spot typically covering 1 to 10 cells (11). As a result, deconvolution algorithms such as Cell2location are required to infer cellular composition (1).
10x Visium HD: This platform significantly improves resolution to near single-cell scale (2 × 2 μm) using a continuous lawn of barcoded squares (1). It provides superior spatial fidelity compared to previous sST platforms and supports FFPE (formalin-fixed, paraffin-embedded) samples through probe-based chemistry and the CytAssist instrument (11). This compatibility with archived tissue collections is key for clinical adoption. Recent benchmarking studies in tumor samples (2025) confirmed that Visium HD achieves better transcript diffusion control than Stereo-seq (1).
Stereo-seq (DNB-based): Uses DNA nanoball (DNB) arrays to achieve subcellular nominal resolution (0.22 μm) (1). Although its theoretical resolution is high, comparative studies show a higher rate of invalid spatial barcodes and transcript diffusion, leading to lower effective fidelity than Visium HD (1). It also requires significantly deeper sequencing coverage (1).
GeoMx DSP: Unlike Visium or Stereo-seq, GeoMx is a region-of-interest (ROI)-based technology that uses digital barcoding to profile mRNA or proteins in predefined tissue regions (1). Each ROI typically contains 50–100 cells, making GeoMx ideal for analyzing rare or spatially dispersed populations, with efficient multiplexing of small samples (1).
Together, these sequencing-based platforms represent the broad-coverage arm of ST, capturing the full transcriptome while trading off spatial precision.
In Situ Imaging-Based Platforms (iST)
In contrast, imaging-based platforms rely on iterative fluorescent probe hybridization (smFISH) to profile preselected gene panels, reaching subcellular or single-molecule resolution (1).
MERFISH / MERSCOPE and seqFISH+: MERSCOPE, based on MERFISH technology, uses binary barcoding for error-robust detection and allows panels of up to 1,000 customizable genes for any species (1).
10x Xenium SMI and NanoString CosMx SMI: These are high-throughput, industry-leading iST platforms supporting large panels of 5,000–6,000 genes (1).
Xenium 5K uses padlock probes with cyclic in situ sequencing, while
CosMx 6K employs branched probes with positional decoding (1).
A systematic benchmarking study in 2025 (COAD, HCC, OV tumors) reported key performance metrics (1):
Xenium 5K demonstrated the strongest correlation with scRNA-seq, higher annotation accuracy, and the lowest background noise, indicating superior specificity.
CosMx 6K detected more total transcripts but showed higher background and lower gene-to-gene variation, limiting its ability to resolve differential expression in complex tissues.
In summary, imaging-based ST platforms achieve unmatched resolution and specificity, making them ideal for characterizing fine tissue niches and cellular microenvironments.
Cellular Segmentation and Spatial Fidelity
The limiting factor in subcellular ST is not theoretical resolution, but rather the ability to control technical artifacts and achieve precise cell segmentation.
Segmentation vs. Spillover: Accurate cellular segmentation is critical to isolate transcripts and prevent signal leakage between adjacent cells. Benchmarking studies revealed that sequencing-based ST (especially sST) often shows artificial co-expression of mutually exclusive markers (for example, EPCAM and CD3E), a limitation largely mitigated in Xenium 5K after segmentation, reaching separation levels comparable to scRNA-seq (1).
Multimodal Segmentation: iST platforms such as Xenium leverage multiple staining channels (DAPI, membrane, cytoplasm) to define cellular boundaries with higher accuracy (1). This capability is especially valuable for complex pathological tissues, such as tumors containing irregularly shaped cells (for example, hepatocytes) or multinucleated cells, which cannot be properly segmented using nucleus-only methods (1).
In practice, spatial fidelity and spillover control are the most critical quality metrics for studying niche biology. Even with reduced gene coverage, high-fidelity iST platforms often outperform WTA-based methods when the biological question depends on precise cell–cell interactions.
The following table summarizes the key technical specifications of high-resolution spatial transcriptomics platforms:
Every platform offers a different lens to observe biology. But the real power of spatial transcriptomics emerges when these views are integrated, when imaging and sequencing converge into a single computational space. The next section examines how data integration transforms isolated observations into coherent biological narratives.
Data Integration and Computational Strategies
The Spatial Multi-Omic Ecosystem
Spatial transcriptomics (ST) serves as the central axis for integrating multiple layers of molecular information within the tissue context, enabling a transition toward a holistic view of biology.
Integration with Proteins:
Simultaneous or sequential protein measurement is essential, since proteins (such as PD-L1 or membrane receptors) are the direct functional effectors and the most established clinical biomarkers. (1)
Platforms such as GeoMx, CosMx, and MERSCOPE already offer multiplexed proteomic profiling, either on the same section or on adjacent serial sections, providing a critical benchmark for validating gene expression data. (1)Integration with Genomics:
Computational methods now allow inference of genomic alterations, such as copy number variations (CNVs), directly from ST data (for example, Cottrazm)(1).
This capability is vital for distinguishing malignant cells from stromal populations and, therefore, for precisely delineating the tumor invasive front (TIF)(1).
Integration of ST with Histological Imaging and Digital Pathology (ST + H&E)
The integration of ST with digital pathology represents the most promising path toward clinical scalability.
Robust Alignment:
The prerequisite for any integration is the accurate registration of the H&E image (morphological layer) with the transcript map (molecular layer)(10).
Without this spatial alignment, multimodal analysis loses biological coherence and interpretability.AI as a Force Multiplier:
Artificial intelligence transforms ST (traditionally an expensive, low-throughput experiment) into a high-yield, data-generating technology.MISO and H&Enium:
These deep learning models, based on contrastive alignment, learn to project morphological features extracted from H&E slides and genetic features derived from ST data into a shared latent space(3).
This alignment enriches image-based representations with transcriptomic context, creating biologically meaningful embeddings(10).Scalable Molecular Prediction:
The primary impact of these foundation models lies in their ability to predict gene expression profiles at near single-cell resolution directly from routine H&E slides(3).
This is critical because ST datasets remain costly and relatively scarce(12).
By training AI on the limited corpus of ST data to act as a molecular translator of H&E morphology, the marginal cost of obtaining a spatial biomarker is reduced dramatically, down to the digital review of an already archived slide.
Transfer Learning Strategies
Spatial transcriptomics and digital pathology exist in a mutually reinforcing ecosystem of transfer learning.
ST provides high-quality molecular and spatial ground truth to train robust AI models. In return, large-scale foundation models in pathology, pre-trained on millions of whole-slide images (WSI), can transfer their learned representations to enhance ST embeddings(10).
This representation alignment approach (for example, H&Enium) improves downstream AI tasks such as accurate cell-type classification and gene expression prediction, offering a validated pathway to enhance the clinical utility of standard H&E slides without requiring expensive sequencing assays for every clinical sample (10).
Impact on Drug Development and Precision Medicine
Spatial transcriptomics (ST) has become an essential instrument for dismantling tumor heterogeneity and the tumor microenvironment (TME), two of the main barriers to therapeutic efficacy and the leading causes of clinical failure. (1)
ST extends the value of genomics beyond mutation identification toward the definition of architectural function, turning tissue organization into a quantifiable determinant of therapeutic response.
Discovery and Validation of Contextual Spatial Biomarkers
Spatial transcriptomics has demonstrated that tissue architecture is a superior predictor of therapy response compared with molecular abundance alone.
Immunotherapy Biomarkers:
The localization and functional state of immune cells are more reliable predictors of response to immune checkpoint blockade (ICB) than their mere abundance. (1)
A striking example is the PD-L1 expression specifically in macrophages, rather than in tumor cells, in melanoma. This spatially resolved feature correlates with improved survival and response to anti–PD-1 therapy, a pattern that conventional bulk IHC cannot resolve. (1)Tertiary Lymphoid Structures (TLS):
TLS are lymphoid aggregates associated with favorable prognosis and better response to ICB. (1)
ST not only detects their presence, but also reveals a 50-gene transcriptomic signature (TLS-50) and deciphers their internal complexity, including the migration of plasma cells toward the tumor along fibroblast tracks mediated by CXCL12 signaling in renal cell carcinoma (RCC). (1)Tumor Invasive Front (TIF):
The TIF represents the niche where malignant cells interact with the surrounding stroma.
When combined with copy number variation (CNV) inference, ST enables the precise delineation of the TIF. (1)
Tumor cells at the invasive margin exhibit epithelial-to-mesenchymal transition (EMT) programs, as well as cell-cycle and angiogenesis signatures, consistently associated with poor prognosis across multiple tumor types. (1)
Together, these examples demonstrate how spatial biomarkers integrate molecular identity and tissue context, offering a multidimensional view of tumor biology that is directly actionable for therapy prediction.
Identification of Cellular Subpopulations and Resistant Niches
Deconstruction of Ligand–Receptor Crosstalk:
Using Optimal Transport–based models (SpaOTsc), ST allows functional analysis of spatially constrained ligand–receptor (L–R) interactions that drive tumor survival and therapeutic resistance. (13)Stromal Interactions (CAFs):
In pancreatic ductal adenocarcinoma (PDAC), ST has identified specific subtypes of cancer-associated fibroblasts (CAFs), such as iCAFs, co-enriched for immunosuppressive and lipid metabolism programs.
In high-grade serous ovarian cancer (HGSC), APOE–LRP5 signaling between tumor cells and proximal CAFs has been identified as a mechanism of chemotherapy resistance. (1)Niche-Specific Targets:
In oncologic neuroscience, spatial profiling of glioblastoma (GBM) has revealed a layered organization of cellular states, where hypoxic niches promote inflammation and angiogenesis.
Furthermore, T-cell exhaustion within the GBM microenvironment has been linked to IL-10 signaling from myeloid cells located adjacent to mesenchymal tumor cells. (1)
By exposing these microarchitectural dependencies, ST enables the rational targeting of resistant niches, a crucial step toward overcoming spatially encoded drug resistance.
Reducing Clinical Failure Risk (Clinical De-risking)
ST plays a pivotal role in reducing the risk of clinical failure, a major factor behind the declining return on investment (ROI) in pharmaceutical R&D. (14)
Functional Target Validation:
ST refines target selection by validating whether a receptor or signaling molecule is not only expressed, but functionally active within paracrine communication inside a resistant niche. (13)
This shifts drug development from a reductionist view toward a systems and microenvironmental biology approach, thereby increasing the likelihood that selected targets will remain relevant in vivo. (13)Patient Selection and Spatial Companion Diagnostics (CDx):
Spatial biomarkers, such as the SpatialScore, which quantifies the relative distance between CD4+ T cells and regulatory T cells (Tregs) in cutaneous T-cell lymphoma, have shown greater predictive power for Pembrolizumab response than traditional biomarkers (IHC, mass cytometry). (1)
This refinement of patient stratification directly enhances the precision and efficiency of clinical trials.
Translational and Industrial Outlook
Beyond its scientific depth, ST provides tangible strategic value for the biopharmaceutical ecosystem.
De-risking R&D Pipelines:
Spatial data enable earlier identification of non-responders, improving trial design and reducing Phase II/III attrition rates.Creation of Proprietary Assets:
Spatial biomarkers and AI-driven contextual models can form the basis of intellectual property portfolios, transforming data into durable competitive advantages.Redefining Clinical Evidence:
By merging mechanistic insight with patient stratification, ST bridges discovery and clinical application, positioning spatial data as the new evidentiary standard in precision medicine.
Spatial biology is thus not merely an experimental tool, it is an infrastructure for translational innovation, where tissue architecture becomes both a biological variable and a strategic resource.
Current Challenges and Economic Realities
Technical and Operational Limitations
Sample Quality and FFPE Compatibility:
Spatial transcriptomics (ST) requires rigorous control of pre-analytical variables. Compatibility with formalin-fixed paraffin-embedded (FFPE) samples is essential for clinical archiving (1), but fixation-induced RNA fragmentation can exacerbate transcript diffusion and compromise spatial precision, particularly in sequencing-based platforms. (1)Complexity and Throughput:
The per-sample cost of ST assays remains high, and the complexity of workflows (especially long imaging and hybridization cycles in imaging-based (iST) platforms) still limits throughput for large-scale translational studies. (1)Lack of Standardization:
There is still no consensus on pre-analytical protocols, such as fixation times, preservation conditions, or quality control metrics. This variability introduces uncertainty in reproducibility across laboratories, a key concern for regulatory-grade clinical trials. (15)
Bioinformatic and Regulatory Challenges
Shortage of Annotated Datasets:
The scarcity of large, expert-annotated ST datasets limits the capacity to train and validate foundation modelscapable of robust knowledge transfer across platforms and tissue types. (8)
Pathologist-curated datasets are indispensable to ensure biological accuracy in AI-driven interpretations of spatial data.Regulatory Translation of Spatial Biomarkers:
The major bottleneck for clinical adoption is the absence of established regulatory frameworks.
Rigorous prospective clinical validation of spatial biomarkers, such as TLS or TIF signatures—and their approval as companion diagnostics (CDx) still face substantial challenges.
Current regulatory pathways are not yet designed to evaluate context-dependent measurements, which combine molecular identity with spatial organization. (1)
The ROI Challenge and Big Pharma Adoption
The pharmaceutical R&D sector faces unprecedented economic pressure: the cost of bringing a new asset to market has doubled, while average return on investment (ROI) has fallen from 10.1% to 1.9% over the past decade. (14)
Integration and Perceived Risk:
ST is often perceived as an expensive, complex technology with uncertain short-term ROI, which limits its adoption in high-throughput industrial pipelines. (1)
Technical variability observed in benchmarking studies (for example, between Xenium and CosMx) further undermines confidence in large-scale reproducibility. (1)Systemic Value as the True Metric:
The real economic value of ST is not measured by cost per sample, but by its ability to mitigate late-stage clinical failure, which represents the most expensive component of drug development. (14)The Systemic Value Approach:
ST enables the rational design of molecules targeting niche-specific functional networks (for example, stromal ligand–receptor interactions) and supports the development of superior spatial companion diagnostics (CDx).
By improving target validation and patient stratification before Phase III, ST directly enhances the overall ROI of pharmaceutical portfolios.
In essence, spatial transcriptomics should not be evaluated as an incremental laboratory technology, but as a strategic risk-reduction infrastructure for pharmaceutical innovation.
Toward a Contextual and Spatial Medicine
Toward the Fourth Dimension (4D Spatiotemporal Omics)
The future of spatial transcriptomics (ST) is oriented toward capturing both temporal and three-dimensional complexity.
3D Biology:
It is imperative to move beyond the analysis of thin two-dimensional sections.
Three-dimensional reconstructions from serial sectioning, as already applied in pancreatic ductal adenocarcinoma (PDAC) and precancerous lesions (1), or the development of new in situ sequencing technologies for thick tissues (such as StellarOmics), now allow the complete mapping of tumor architecture and niche dynamics. (1)Temporal Dynamics (4D):
Integrating ST data with RNA velocity and pseudotime analysis enables inference of cellular state transitions, including clonal evolution and the progression from precancerous to invasive lesions, directly within the spatial context of the tissue. (1)
Together, these advances point toward a 4D spatiotemporal understanding of tissue biology, where structure, function, and time are captured as a unified system.
How ST Redefines the Notion of “Tissue Intelligence”
Tissue intelligence can be defined as the emergent functional property of an organ or tumor resulting from the architectural organization and coordinated communication of its cellular components.
Spatial transcriptomics is the primary tool for decoding this intelligence, transforming pathology from a descriptive discipline (focused on morphology) into a systems-engineering discipline that analyzes functional spatial networks.
Artificial intelligence, guided by ST, enables the reconstruction of tissue structure and the tumor microenvironment by leveraging higher-order relationships between adjacent tissue programs. (16)
This integration allows us to move from static observation to dynamic understanding, revealing how local interactions give rise to global biological behavior.
Future Potential for Integrative Foundation Models
The next generation of Foundation Models (FMs) will be designed to natively integrate the three fundamental domains of biomedical information:
Genomics (mutations, copy number variations)
Histology/Imaging (H&E, WSI)
Spatial and Proteomic Biology (ST, spatial proteomics) (9)
These multimodal foundation models will deliver robust predictions, biologically interpretable biomarkers, and cross-domain validation, enabling treatment personalization at a contextual level never before achieved.
AI will identify biomarkers that, by integrating multiple data layers, overcome the limitations inherent to any single-omic measurement, bridging morphology, expression, and function within a unified spatial framework.
Spatial transcriptomics is the roadmap that transforms linear biology into functional ecology, laying the foundation for a truly contextualized precision medicine, one that can, for the first time, target not only the diseased cell but also the systemic environment that sustains it.
References:
Gong D, Arbesfeld-Qiu JM, Perrault E, Bae JW, Hwang WL. Spatial oncology: Translating contextual biology to the clinic. Cancer Cell. 2024 Oct;42(10):1653–75.
Wang Y, Liu B, Zhao G, Lee Y, Buzdin A, Mu X, et al. Spatial transcriptomics: Technologies, applications and experimental considerations. Genomics. 2023 Sept;115(5):110671.
Schmauch B, Herpin L, Olivier A, Duboudin T, Dubois R, Gillet L, et al. A deep learning-based multiscale integration of spatial omics with tumor morphology [Internet]. bioRxiv; 2024 [cited 2025 Oct 24]. p. 2024.07.22.604083. Available from: https://www.biorxiv.org/content/10.1101/2024.07.22.604083v1
Luo J, Fu J, Lu Z, Tu J. Deep learning in integrating spatial transcriptomics with other modalities. Brief Bioinform. 2025 Jan 1;26(1):bbae719.
Mo Y, Liu J, Zhang L. Deconvolution of spatial transcriptomics data via graph contrastive learning and partial least square regression. Brief Bioinform. 2025 Feb 10;26(1):bbaf052.
Slabowska AO, Pyke C, Hvid H, Jessen LE, Baumgart S, Das V. A systematic evaluation of state-of-the-art deconvolution methods in spatial transcriptomics: insights from cardiovascular disease and chronic kidney disease. Front Bioinform. 2024 Mar 27;4:1352594.
Mapping human lymph node cell types to 10X Visium with Cell2location [Internet]. Mapping human lymph node cell types to 10X Visium with Cell2location. Available from: https://cell2location.readthedocs.io/en/latest/notebooks/cell2location_tutorial.html
SPADE: Spatial Transcriptomics and Pathology Alignment Using a Mixture of Data Experts for an Expressive Latent Space [Internet]. [cited 2025 Oct 24]. Available from: https://arxiv.org/html/2506.21857v1
Wang C, Cui H, Zhang A, Xie R, Goodarzi H, Wang B. scGPT-spatial: Continual Pretraining of Single-Cell Foundation Model for Spatial Transcriptomics [Internet]. bioRxiv; 2025 [cited 2025 Oct 24]. p. 2025.02.05.636714. Available from: https://www.biorxiv.org/content/10.1101/2025.02.05.636714v1
Glettig M, Ehrensperger T, Yates J, Boeva V. H&Enium, Applying Foundation Models to Computational Pathology and Spatial Transcriptomics to Learn an Aligned Latent Space [Internet]. bioRxiv; 2025 [cited 2025 Oct 24]. p. 2025.07.22.665986. Available from: https://www.biorxiv.org/content/10.1101/2025.07.22.665986v1
Glettig M, Ehrensperger T, Yates J, Boeva V. H&Enium, Applying Foundation Models to Computational Pathology and Spatial Transcriptomics to Learn an Aligned Latent Space [Internet]. bioRxiv; 2025 [cited 2025 Oct 24]. p. 2025.07.22.665986. Available from: https://www.biorxiv.org/content/10.1101/2025.07.22.665986v1
Wang H, Cheng P, Wang J, Lv H, Han J, Hou Z, et al. Advances in spatial transcriptomics and its application in the musculoskeletal system. Bone Res. 2025 May 16;13:54.
Cao J, Li C, Cui Z, Deng S, Lei T, Liu W, et al. Spatial Transcriptomics: A Powerful Tool in Disease Understanding and Drug Discovery. Theranostics. 2024 May 11;14(7):2946–68.
MedPath [Internet]. 2020 [cited 2025 Oct 24]. Digital Technologies Poised to Reverse Declining ROI in Pharmaceutical R&D. Available from: https://trial.medpath.com/news/488759d89d39a281/digital-technologies-poised-to-reverse-declining-roi-in-pharmaceutical-r-d
Smith KD, Prince DK, MacDonald JW, Bammler T, Akilesh S. Challenges and Opportunities for the Clinical Translation of Spatial Transcriptomics Technologies. Glomerular Dis. 2024 Mar 13;4(1):49–63.
Spatial resolved transcriptomics: Computational insights into gene transcription across tissue and organ architecture in diverse applications [Internet]. [cited 2025 Oct 24]. Available from: https://www.the-innovation.org/article/doi/10.59717/j.xinn-life.2024.100097



Excellent analysis! I love how you highlight the crucial role of spatial context. It realy resonates with how we approach data in AI, where understanding relationships is key. This shift from linear to ecological thinking is so important for redefining biology. Such insightful work.