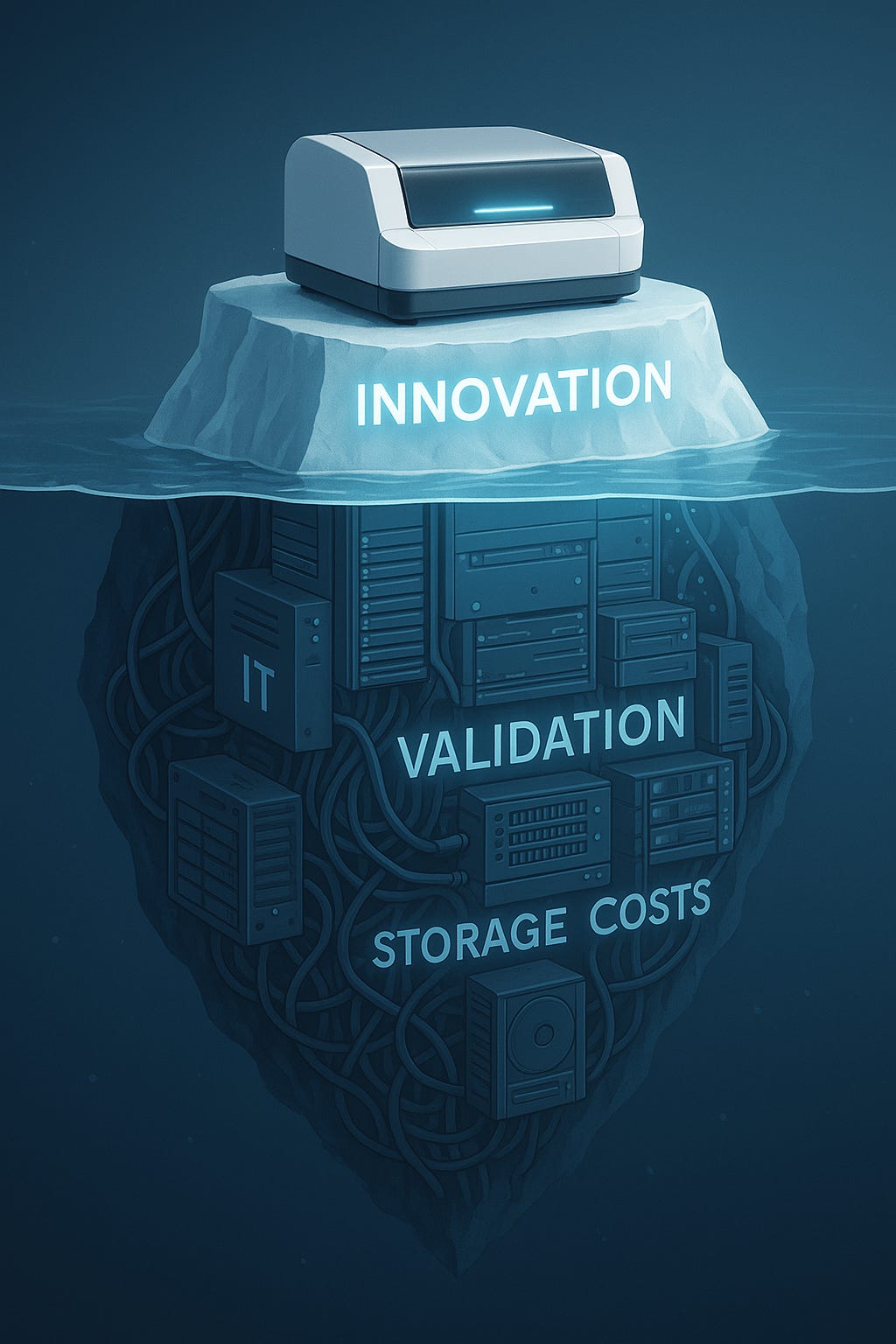
Digital Pathology starts with the scanner: A Strategic Perspective
For more than a century, pathology has relied on one tool above all others: the optical microscope. Slide after slide, pathologists have peered into glass to unlock the secrets of disease. It’s a method that has stood the test of time, reliable, precise, even iconic. But it also comes with limits that are harder and harder to ignore: slow workflows, little scalability, and collaboration that too often stops at the edge of the lab.
Those cracks in the system are widening. Across the globe, there are fewer pathologists available just as case volumes are exploding. Precision medicine is demanding more complex answers from tissue than ever before. And hospitals are under pressure to connect pathology data with radiology, genomics, and clinical records to see the whole patient, not just a slice of tissue under glass. The microscope alone cannot carry that weight.
Enter digital pathology. At the heart of this shift is one piece of technology that changes everything: the whole slide imaging (WSI) scanner. It’s not just a digital stand-in for the microscope. It’s the gateway to an entirely new ecosystem of possibilities. A scanner transforms a physical slide into a digital asset, something that can be stored, shared, analyzed by algorithms, and integrated into the broader world of precision medicine.
Scanners unlock telepathology. They feed artificial intelligence. They allow a biopsy taken in a rural hospital to be reviewed in real time by a subspecialist hundreds of miles away. In short: they turn glass into data, and data into decisions.
So while it’s tempting to focus the conversation on AI and biomarkers, the truth is that none of that matters without this first step. The scanner isn’t a background tool, it’s the catalyst. And how we choose to embrace it will define not only the future of pathology, but also who gets access to the promise of precision medicine.
1. The Fundamental Role of the Scanner in Digital Pathology
The journey from analog to digital pathology begins at one decisive moment: when a glass slide is transformed into a digital asset. That step is made possible by the whole slide imaging (WSI) scanner, a device that doesn’t just take pictures, but becomes the very entry point into the digital pathology ecosystem. Its role goes far beyond image capture: it creates the foundation on which efficiency, collaboration, and diagnostic innovation are built.
1.1 From Anatomy to Data Capture: How WSI Scanning Works
Whole slide imaging is a sophisticated engineering process that brings together several precision components to digitize an entire histology or cytology specimen. Unlike a snapshot of a single field of view, the scanner replicates the full experience of navigating a glass slide under the microscope, but in a digital environment.
At its core, the process relies on three key elements:
High-Quality Optics: Using microscope-grade objectives at 20x or 40x, the scanner captures subtle cellular and morphological details, the kind that often make the difference in a diagnosis.
Precision Motorized Stage: The glass slide rests on an automated platform that moves systematically across the tissue, ensuring every region is imaged and later stitched seamlessly together.
High-Resolution Camera: The “heart” of the scanner. Instead of one image, it captures thousands of magnified snapshots in rapid succession, completing the process in just minutes.
Once captured, specialized software takes over. It stitches those thousands of images into a flawless mosaic, producing a single high-resolution digital file. Formats like TIFF or SVS ensure long-term preservation with full fidelity to the original specimen. In essence, the scanner transforms fragile glass into a durable, shareable, and permanent digital record.
1.2 The Scanner as a Catalyst for Operational Transformation
Scanning isn’t just a digital substitute for the microscope. It’s the engine behind a deeper operational transformation, one that addresses inefficiencies ingrained in traditional pathology. By turning physical information into digital data, scanners unleash a cascade of benefits that streamline workflows and ultimately improve patient outcomes.
Take efficiency. High-capacity scanners, such as the Philips SG300, can digitize hundreds of slides autonomously, even overnight. That automation frees up lab technicians from repetitive manual handling, allowing them to focus on higher-value tasks. It also cuts turnaround times dramatically by removing the logistical bottlenecks of moving glass slides around.
Beyond the lab walls, scanners are the enablers of telepathology and global collaboration. In the analog world, seeking a second opinion meant shipping glass slides across cities (or even countries) at great cost and risk, often waiting days or weeks for results. Digital slides erase those delays. With a secure internet connection, cases can be shared instantly for real-time consultation. For rural and underserved areas, this capability can mean access to expertise that would otherwise be out of reach.
And then there’s artificial intelligence. Digital slides are the raw material for computational pathology. Algorithms can quantify mitotic rates, measure biomarker expression, detect micrometastases, or highlight anomalies that might otherwise go unnoticed. These tools don’t replace the pathologist, they extend our reach, acting as “augmented intelligence” that can triage urgent cases and bring consistency to complex evaluations.
In many ways, the scanner is the data factory of digital pathology. It’s where glass becomes data, and data becomes an asset, one that can be stored, analyzed, shared, and even monetized for research and development. The ability to capture high-fidelity information at scale is what makes deep learning possible, and what positions pathology to finally integrate seamlessly into the broader world of precision medicine.
2. Challenges to Adoption: Beyond the Price Tag
For all its promise, the adoption of digital pathology has been anything but straightforward. The obstacles run deeper than the sticker price of a scanner. What we face instead is an ecosystem of barriers, economic, technical, regulatory, and cultural, that must be addressed together if digital pathology is to move from niche adoption to widespread reality.
2.1 The Economic Barrier: A Web of Hidden Costs
It’s tempting to think cost is the main barrier. But that’s an oversimplification. Yes, a high-capacity scanner can range anywhere from $100,000 to $500,000, though the market now offers lower-end models for as little as $2,000. The real challenge lies in what comes after the purchase.
The biggest “hidden cost” is the IT infrastructure needed to handle the massive flow of data. A single whole slide image can easily exceed 1 GB, and a high-volume lab can generate hundreds of terabytes each year. Managing that flood requires serious investment:
High-speed networks: To navigate digital slides without frustrating delays, 10 Gb/s connectivity is often recommended.
Massive, redundant storage: Labs must keep years’ worth of slides for legal and clinical review. That means scalable storage, whether on local servers or the cloud.
Visualization hardware: Pathologists need 4K monitors and powerful workstations to explore slides with the same confidence they have under the microscope.
And that’s just capital expenditure. Operating costs keep adding up: annual software licenses, hardware maintenance contracts, and perhaps most critically, the IT personnel needed to manage, secure, and support this infrastructure. For many institutions, these expenses become the real bottleneck.
2.2 Regulatory and Quality Hurdles
Even when the technology performs brilliantly, trust is not automatic. For pathologists, image quality is non-negotiable. Modern scanners produce stunning resolution and color fidelity, but doubts linger: can digital images truly capture the subtle nuances of tissue that our eyes detect through the microscope?
To address that skepticism, validation is mandatory. Organizations such as the College of American Pathologists (CAP) have set clear guidelines, recommending validation with at least 60 cases per application, comparing diagnoses between glass and digital. It’s a rigorous, time-consuming, and costly process, but an essential one. Without it, confidence falters, and adoption stalls.
2.3 Resistance to Change and the Knowledge Gap
Not all barriers are financial. Some are cultural and perhaps even emotional. The microscope has been the symbol of pathology for over a century. For many pathologists, trading the tactile familiarity of glass and lenses for a mouse and a screen feels like stepping into unfamiliar territory. It’s more than a workflow change; it’s a shift in professional identity.
On top of that cultural resistance, there’s a “digital gap” in understanding the true demands of IT. A pathologist might see the value of scanning slides but underestimate the complexity of managing petabytes of data or the need for high-speed networks to access them fluidly. That lack of awareness can lead to poor planning, underinvestment, and eventual frustration.
In reality, these barriers are intertwined. Economic constraints fuel cultural hesitation; regulatory requirements add uncertainty; and the absence of robust IT strategies magnifies it all. Breaking the cycle requires more than just technology. It calls for change management: ongoing training, clear communication of benefits, and involving pathologists themselves in every step of the digital transition. Only then can adoption move from resistance to momentum.
3. Innovative Models for Democratizing Access
The barriers to adoption are real, but they’re not insurmountable. Across the industry, new business models are emerging that break the link between digitalization and hardware ownership. Instead of forcing every institution to shoulder the full cost of scanners and infrastructure, these approaches offer flexible alternatives tailored to different needs and budgets. The result is a more inclusive path to digital pathology one where access isn’t reserved only for the best-funded labs.
3.1 Scanning-as-a-Service
One of the most effective solutions to the capital cost barrier is the Scanning-as-a-Service (SaaS) model. Rather than buying a scanner and building the infrastructure to support it, institutions outsource the digitization and hosting of slides to an external provider.
The model transforms a heavy upfront investment (CAPEX) into a predictable operating expense (OPEX). That shift makes digital pathology financially viable for smaller labs, research centers, or pharmaceutical companies with variable case volumes. The service provider takes on the responsibility for purchasing the scanner, maintaining it, and managing the complex IT backbone storage, data security, and system uptime included.
For the end user, the cost is simply a subscription or a per-slide fee. No massive investment, no looming risk of technological obsolescence. By outsourcing the “data factory,” even smaller institutions can access the benefits of telepathology and AI without building their own digital infrastructure from scratch.
3.2 Hub-and-Spoke Pathology Networks
Another model, inspired by logistics and healthcare delivery, is the hub-and-spoke approach. Here, a centralized high-volume laboratory (the hub) handles most of the scanning and case analysis, while smaller labs (the spokes) focus on urgent or on-site needs.
Digital pathology makes this model possible at scale. Instead of shipping glass slides across the country, digital images move instantly from spokes to the hub, where subspecialists can review them in real time. A single pathologist at the hub can support multiple remote sites, helping to close gaps caused by workforce shortages and geographic inequality.
The effect is profound: distance no longer dictates access. What once required expensive courier services and days of waiting can now be achieved with a secure connection and a few clicks. For rural or underserved regions, this model turns digital pathology into a direct solution for equitable diagnostic care.
3.3 Hybrid Solutions and Low-Cost Scanners
For many institutions, the most pragmatic path is a hybrid one. Instead of choosing between a full in-house system or complete outsourcing, they combine both.
A hospital, for instance, might acquire a smaller, lower-cost scanner like the Philips SG60 or a single-slide model to digitize its daily caseload. Those images are stored locally for fast, seamless access. Meanwhile, long-term archiving is handled through a cloud service, eliminating the need for expensive local data centers.
This blended approach strikes a balance: it lets institutions scale storage gradually, avoid unpredictable “data egress” fees often tied to cloud providers, and keep the most frequently accessed data close at hand. At the same time, the cloud offers flexibility and security for long-term retention. In short, hybrid models make it possible to enjoy the best of both worlds, local speed and control, paired with the scalability of cloud infrastructure.
4. Real-World Examples of Implementation
Theory is important, but nothing speaks louder than lived experience. Around the world, pioneering institutions have shown not only that digital pathology works, but also how it can be implemented thoughtfully to overcome barriers and deliver real value. These case studies offer lessons that are as practical as they are inspiring.
4.1 Case Studies from the Developed World
In Portugal, IMP Diagnostics provides a striking example of a careful, strategic transition. Their team resisted the temptation to jump headfirst into digital pathology. Instead, they conducted extensive due diligence: visiting other labs, closely tracking the evolution of the technology, and waiting for the right moment.
What tipped the balance? The maturation of AI algorithms, the publication of credible clinical evidence, FDA regulatory approval for diagnostic use, and the availability of next-generation high-performance scanners. By moving deliberately, IMP ensured that the technology was ready to meet the demands of a real clinical workflow. Their story underscores a critical point: success in digital pathology is not about speed, but about timing and strategic planning.
The UK’s National Health Service (NHS) offers a different kind of lesson, one at scale. Its ambitious plan to consolidate pathology services into 29 hub-and-spoke networks aims to achieve massive efficiency gains, cut costs, and standardize care nationwide. But such scale brings its own challenges: integrating IT infrastructures, managing logistics, and addressing staff resistance to relocation or workflow changes. The project shows both the promise and the complexity of change management in digital pathology. Tackling these hurdles head-on is essential for long-term success.
4.2 Latin American Pioneers: Closing the Digital Divide
Digital pathology is not the exclusive domain of wealthy economies. Across Latin America, institutions are adopting it as a pragmatic solution to geographic barriers and specialist shortages.
In Peru, IREN Norte has implemented telepathology for cancer diagnostics, enabling oncologic cases to be reviewed remotely. In Colombia, Salud Colsubsidio announced the country’s first fully digital pathology model, an initiative that signals a shift in how diagnostics can be delivered in resource-constrained settings. Both examples show how digital pathology can become a lifeline for patients in underserved regions.
Brazil provides perhaps the most powerful case. The diagnostic giant DASA leveraged Google Cloud infrastructure for genomic and pathology data processing. The result? A 90% reduction in infrastructure costs. For a region where resources are limited and demand is high, this case demonstrates how cloud technology can make digital pathology not only possible, but sustainable.
Together, these stories highlight an essential truth: digital pathology is not a privilege, it’s a necessity. And with the right models and partnerships, it can be scaled to serve both advanced healthcare systems and regions where access to diagnostics has long been inequitable.
5. Strategic Perspective: The Future of the Scanner and Precision Medicine
The true promise of the scanner in digital pathology goes far beyond saving costs or streamlining workflows. The scanner is the engine of a much deeper transformation: it’s the key to embedding pathology into the very fabric of precision medicine and integrated diagnostics. In doing so, it elevates its strategic value and unlocks a far broader return on investment (ROI).
5.1 The Scanner as a Catalyst for Expansive ROI
When evaluating the ROI of digital pathology, it’s important to take a holistic view. Yes, the tangible savings in courier costs, physical storage, and staff time are significant. But the real value lies elsewhere: in reducing diagnostic errors, improving quality, and creating entirely new business opportunities.
ROI in digital pathology isn’t just a financial calculation. It’s a mix of operational efficiency and strategic advantage that strengthens an institution’s position for the long term.
Digitization also opens the door to data monetization. The ability to scan and archive vast volumes of slides creates valuable collections digital biobanks. These datasets, once anonymized, can be licensed to pharmaceutical and biotech companies to develop and train AI algorithms. This not only generates new revenue streams but also contributes directly to the advancement of medical research.
5.2 Towards Full Integration: The Scanner at the Center of the Data Ecosystem
For too long, pathology has operated in isolation. The knowledge locked in glass slides has remained separate from radiology, genomics, and electronic health records. The scanner breaks down those walls. By converting slides into high-fidelity digital assets, it transforms pathology into structured data data that can be integrated seamlessly with other diagnostic sources.
This multimodal integration paves the way for what many call the digital twin of the patient: a virtual model that combines pathology, radiology, and genomics to predict disease progression and treatment response. It’s here that AI reveals its true power drawing insights from the interplay of multiple data streams and supporting clinicians with evidence-based predictions.
In this vision, the scanner is not just a tool for digitization; it is the central piece of infrastructure that enables faster, more accurate, and more personalized diagnosis. It positions pathology not at the margins but at the very center of precision medicine, working hand in hand with other disciplines to deliver care that is truly integrated.
Conclusion: The Scanner as the Engine of Pathology’s Evolution
Digital pathology is not a passing trend it is the inevitable evolution of a vital discipline. At the heart of this transformation lies the whole slide scanner: the essential starting point that turns the physical world into a digital data ecosystem, unlocking operational and diagnostic benefits that the traditional microscope simply cannot offer.
Yes, the upfront investment and the barriers to adoption (hidden infrastructure costs, entrenched cultural resistance) are significant. But the path forward is already visible. Scanning-as-a-Service makes digital pathology accessible to smaller labs by turning heavy capital investment into manageable operational expense. Hub-and-spoke networks prove that digital pathology can directly address workforce shortages and geographic inequities. And hybrid solutions offer a pragmatic middle ground, balancing local control with the scalability and flexibility of the cloud.
For healthcare leaders, the question is no longer if to digitalize, but how to do it wisely. Successful implementation is not just about buying a scanner; it’s about embracing a vision that sees the scanner as more than hardware, as a data engine that breaks silos and brings pathology into the center of truly collaborative precision medicine.
By investing not only in scanners but in the ecosystem that surrounds them, institutions stand to gain more than efficiency. They help build a healthcare system that is more equitable, standardized, and ultimately more beneficial to patients. That is the real promise of digital pathology and it begins with the scanner.